MicrobeCare : Independently, Clinically Studied
MicrobeCare has been clinically studied in the American Journal of Infection Control by leading infection control epidemiologists and has shown persistence in preventing the rebound effect of microorganisms.

We found that a single application of MicrobeCare™, [provides] a significant and persistent, long-term reduction in O.R. surface contamination.”
Dr. Charles E. Edmiston Jr.
PhD, SM (ASCP), CIC (CBIC), FIDSA, FSHEA, FAPIC
Emeritus Professor of Surgery, Medical College of Wisconsin
Operating Room Study
4 operating rooms in a medical center were sampled three times over a 6-week period. The operating rooms included two general surgical O.R., a hybrid O.R. where open and endovascular procedures are performed, and an O.R. used for kidney and liver transplants. The O.R.’s were used as normal during the 6-week study.
100% of terminally cleaned O.R. surfaces tested culture-positive with heavy bacterial contamination before being treated with MicrobeCare™
82.5% of O.R. surfaces tested were culture-negative 6-weeks after treatment.
Of the surfaces that were culture
positive, the mean colony count after 6-weeks treated surfaces = 0.8 cfu
untreated surface = 14.3 cfu
73% reduction in ATP scores 6-weeks after treating O.R. surfaces with
MicrobeCare™
mean ATP score after 6-week;
treated surfaces = 75.9 RLU
untreated surfaces = 279.9 RLU
Selective high touch surfaces in a 26-bed medical intensive care unit at a tertiary medical center were sampled over a 6-week period. The sample sites included telephone handpieces, computer keypads, surfaces of physician workstations, and selective patient items (blood pressure cuffs and patient bed tables).
97.5% of surfaces were assessed as “dirty” before being treated with MicrobeCare™
95.5% of I.C.U. surfaces tested culture-negative 6-weeks after treatment,
Of the surfaces that were culture positive, the mean colony count after 6-weeks treated surfaces = 0.83 cfu untreated surfaces = 51 cfu
85% reduction in ATP scores 6-weeks after treating high touch I.C.U. surfaces with
mean ATP score after 6-weeks;
treated surfaces = 44.7 RLU
untreated surfaces = 327.6 RLU
MicrobeCare : Independently, Clinically Studied
Phase 1
The test was set up to verify the effectiveness of MicrobeCare activated on plastic surfaces and then exposed 1200 Cleaning Cycles of Hospital Disinfectant, simulating 12 years of cleaning. Five different disinfectant that are regularly used in hospitals were chosen to use when wiping the samples in the aging process.
Next, the samples were sent to NAMSA for Phase 2, further biological testing to ensure the maximum time the antimicrobial will be effective.
To simulate up to 12 years of cleaning the surface of 5 plastic plates commonly used in operating rooms. This test will verify the effectiveness of the MicrobeCare solution activated on plastic surfaces and then exposed to various industrial cleaners over a period of time.
The samples went through an accelerated aging process that involved wiping the samples 100 to 1200 times with five different cleaners that are regularly used in hospitals. This is equivalent to 12 years of cleaning at a hospital. Next, the samples were sent to NAMSA for further biological testing to ensure the maximum time the antimicrobial will be effective.
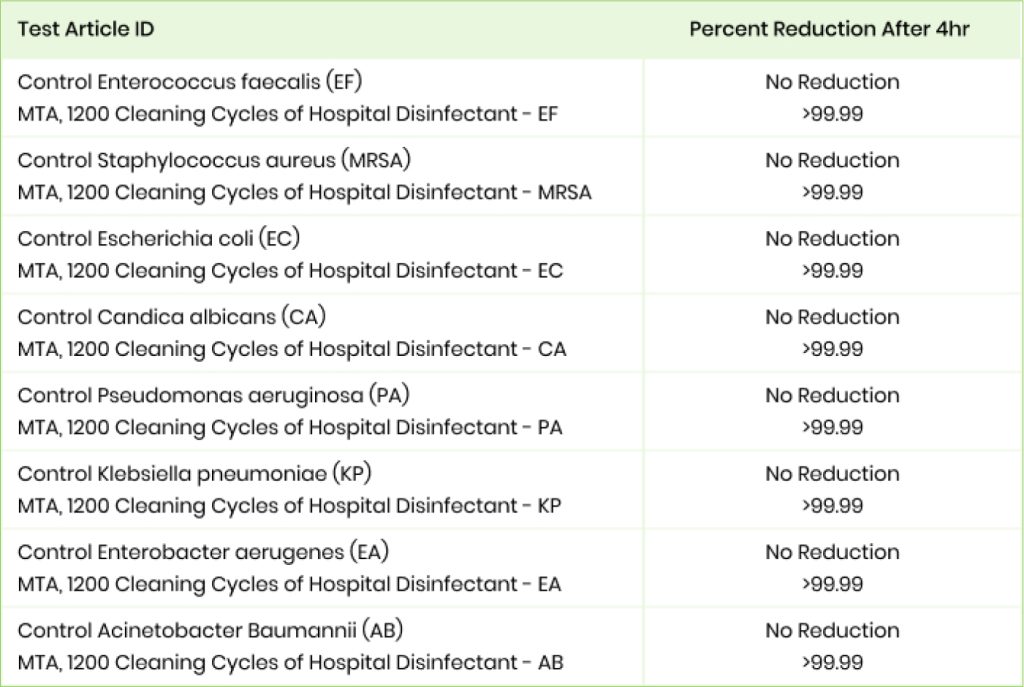
Phase 2
Purpose : To test the efficacy of MicrobeCare against an array of bacteria after undergoing a 12 year simulated cleaning cycle.
Process : The plates were contaminated with several microorganisms including E-coli and MRSA and then tested 4 hours later for contamination.
Results : All 4 plates treated with MicrobeCare showed >99.99% reduction in contamination.